Background: Motixafortide is a novel, high affinity CXCR4 antagonist with extended receptor occupancy that facilitates the migration of hematopoietic stem and progenitor cells from the bone marrow into the peripheral blood in preparation for autologous stem cell transplantation (ASCT). In prior pre-clinical and clinical studies, motixafortide was associated with transient hypersensitivity and injection site reactions. Strategies to mitigate such adverse events (AEs) commonly include agents with antihistamine activity. The Phase 3 GENESIS trial demonstrated that 88.8% of Multiple Myeloma patients mobilized ≥6x10 6 CD34+ cells/kg (local labs) in 1 leukapheresis procedure (LP) following the administration of 1 dose of motixafortide in combination with GCSF. Per initial protocol, premedication with an antihistamine H1 blocker prior to motixafortide administration was provided to all patients. However, during the course of the GENESIS study a protocol amendment was implemented to include premedication with an antihistamine H1 blocker, an H2 blocker, a leukotriene inhibitor and acetaminophen prior to motixafortide administration. Here we report the safety findings in a cohort of patients at who received single drug premedication with an H1 antihistamine prior to the protocol amendment and those who received the updated combination pre-medication regimen following protocol amendment.
Methods:The GENESIS trial was a multicenter, randomized, double-blind, placebo-controlled study in 122 transplant-eligible multiple myeloma patients, as previously described (PMID: 37069359). Included in this analysis are 46 patients enrolled at the Washington University in St. Louis; 32 patients were randomized to the motixafortide treatment arm and 14 patients were randomized to the placebo arm. Among motixafortide-treated patients, 22 received a single-agent H1 blocker pre-medication and 10 received a combination pre-medication regimen comprising of an H1 blocker, an H2 blocker, a leukotriene inhibitor and acetaminophen. All pre-medications were administered within 1 hour prior to motixafortide administration. Hypersensitivity AEs included all events coded to the MedDRA terms of urticaria, pruritus, flushing, erythema, rash, hypersensitivity or anaphylaxis. Injection site reactions included all events coded to the MedDRA High Level Term of Injection Site Reaction. All AEs were graded using CTCAE criteria and were recorded up to 30 days from the last dose of motixafortide.
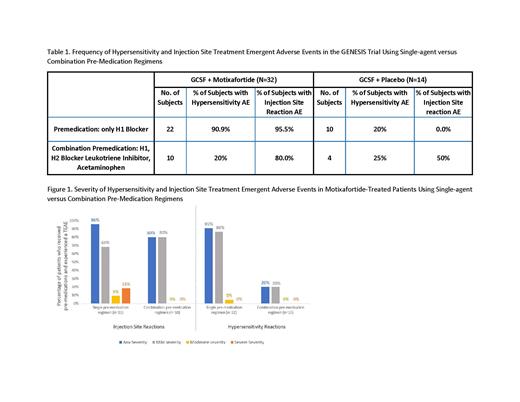
Results: Overall, motixafortide was safe and well tolerated with no episodes of anaphylaxis, no Grade 4 AEs and no deaths. The most commonly observed AEs included transient hypersensitivity and injection site reactions. The frequency of motixafortide-associated hypersensitivity AEs of any grade was reduced from 90.9% with single-agent H1 blocker pre-medication to 20% with the combination pre-medication regimen, similar to the 20-25% rate of hypersensitivity AEs reported in the placebo + GCSF cohort (Table 1). In addition, all hypersensitivity AEs that occurred following combination pre-medication were graded mild in severity (Figure 1). Meanwhile the frequency of injection site AEs of any grade was reduced from 95.5% with single-agent H1 blocker pre-medication to 80.0% with the combination pre-medication regimen (Table 1). Furthermore, a notable improvement in severity of injection site AEs was observed, with moderate-severe injection site AEs reduced from 27% with single-agent H1 blocker pre-medication to 0% with the combination pre-medication regimen (Figure 1).
Conclusions:Motixafortide was safe and well tolerated with no episodes of anaphylaxis, no Grade 4 AEs and no deaths. Following implementation of a protocol amendment using combination pre-medication with an antihistamine H1 blocker, an H2 blocker, a leukotriene inhibitor and acetaminophen, the frequency and severity of hypersensitivity and injection site AEs associated with motixafortide were markedly improved.
Disclosures
Crees:BioLineRx, Ltd.: Other: Advisory Board, Research Funding. Stockerl-Goldstein:Celgene: Consultancy. Schroeder:Marker Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sorrento therapeutics: Membership on an entity's Board of Directors or advisory committees; Kura: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; Novo nordisk: Consultancy; Incyte: Honoraria. Vij:Karyopharm: Honoraria; Harpoon: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Legend: Honoraria; Janssen: Honoraria; Pfizer: Honoraria. Sorani:Biolinerx Ltd.: Current Employment, Current equity holder in publicly-traded company. Gliko Kabir:BioLineRx Ltd.: Current Employment, Current equity holder in publicly-traded company. Goldstein:BioLineRx Ltd.: Current Employment, Current equity holder in publicly-traded company. Lustig:BioLineRx Ltd.: Current Employment, Current equity holder in publicly-traded company. Kadosh:BioLineRx Ltd.: Consultancy. DiPersio:Amphivena Therapeutics: Research Funding; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; Sun Pharma Ltd.: Membership on an entity's Board of Directors or advisory committees; Vertex: Consultancy; Magenta: Other: Ownership Investment, Patents & Royalties; Washington University: Current Employment; WUGEN: Other: Ownership Investment, Patents & Royalties; Macrogenics: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; NeoImmune Tech: Consultancy; BiolineRx Ltd: Consultancy, Research Funding; RiverVest Venture Partners: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal